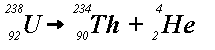
Suppose we have a radioactive decay process of the general form
A --> products
This means that each atom of the radioactive substance A breaks down into a collection of smaller atoms and/or subatomic particles. Phenomena as diverse as dating ancient artifacts and assessing the dangers of nuclear wastes depend on knowing the rate of decay for such reactions.
Here are two examples of specific radioactive decay processes:
(a) 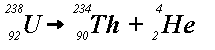
(b)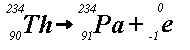
In both cases, the notation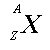 means
X
is the chemical symbol for the element, A is the number of nucleons
(protons and neutrons), and Z is the number of protons. Example
(a) indicates that uranium-238 releases an alpha particle (i.e., a helium
nucleus) to produce thorium-234. This is called alpha-decay. Example
(b) shows that thorium-234 releases a beta particle (i.e., an electron)
to produce protactinium-234. This is called beta-decay. Unstable
nuclides, such as uranium-238, start series of disintegrations that continue
until a stable nucleus results. Mass is lost in both alpha and beta decay
processes. This mass is converted into energy: radiation.
means
X
is the chemical symbol for the element, A is the number of nucleons
(protons and neutrons), and Z is the number of protons. Example
(a) indicates that uranium-238 releases an alpha particle (i.e., a helium
nucleus) to produce thorium-234. This is called alpha-decay. Example
(b) shows that thorium-234 releases a beta particle (i.e., an electron)
to produce protactinium-234. This is called beta-decay. Unstable
nuclides, such as uranium-238, start series of disintegrations that continue
until a stable nucleus results. Mass is lost in both alpha and beta decay
processes. This mass is converted into energy: radiation.
In 1903, Rutherford and Soddy, in a paper entitled Radioactive Change, proposed the law of radioactive change. They observed, in every case they investigated, that the rate of decay of radioactive matter was proportional to the amount present. If we write (as chemists do) [A] for the concentration of the substance A, then the law of radioactive change becomes
rate of change of [A] = -k [A].
The value of k is called the decay constant for A.
This law can be justified in the following way: For any fixed time interval, there is a certain probability (a number r between 0 and 1) that each atom of the radioactive substance will disintegrate. Thus, during this time interval, we should expect that the number of atoms that actually do disintegrate is r times the number present at the start of the interval. That's equivalent to saying that the rate of change of [A] is proportional to the amount present.
We will discuss this "rate
of change" relationship in much greater detail later. Nevertheless,
the functions that have rate of change proportional to the amount present
(exponential functions) have the form
y = y0bt
for some base b,
where y0 is the starting amount. Thus, we would expect
that a concentration function for a radioactive substance has the form
[A](t) = [A]0bt
where b is a number between 0 and 1 (in order to have decay rather than growth), and [A]0 is the starting concentration.
There are several quantitative aspects of radioactivity that are important for managing nuclear waste materials, including how much radioactivity a given substance emits, how concentrated the substance is in its surrounding medium, how dangerous its emissions are to human and other biological populations, and how long the substance goes on being radioactive. In this module we will address only the last of these issues, the time that a given substance remains dangerous.
Part 2: Rapid Decay
In order to determine a
half-life directly from obervational data, the radioactive substance must
decay rapidly enough that one can actually measure a change. Not surprisingly,
the earliest radioactive substances studied were ones that decayed fairly
rapidly. The data in the following table are from a paper published by
Meyer and von Schweidler in 1905. Their numbers are not concentrations
but rather measurements of "relative activity." Since the rate of radioactive
emission is proportional to the amount present, one can measure amounts
(relative to the starting amount) by observing instead the emission rates
(relative to the starting rate).
| Time (days) | Relative Activity |
| 0.2 | 35.0 |
| 2.2 | 25.0 |
| 4.0 | 22.1 |
| 5.0 | 17.9 |
| 6.0 | 16.8 |
| 8.0 | 13.7 |
| 11.0 | 12.4 |
| 12.0 | 10.3 |
| 15.0 | 7.5 |
| 18.0 | 4.9 |
| 26.0 | 4.0 |
| 33.0 | 2.4 |
| 39.0 | 1.4 |
| 45.0 | 1.1 |
Source: S. Meyer and E. von Schweidler, Sitzungberichte der Akademie der Wissenschaften zu Wien, Mathematisch-Naturwissenschaftliche Classe, p. 1202 (Table 5), 1905, as reported in J. Tukey, Exploratory Data Analysis, Addison-Wesley, 1977.
y = y0bt,
with b < 1? If so, can you determine either y0 or b directly from either the graph or the data table?
Y = mx + c
that appears to fit the data in this plot. (You may use the calculator to find the "best fit" line, but do the estimate first. The calculator will use the symbols Y=ax+b, but the number b may of course be different from the "b" in the exponential formula above.)
(it is possible to get the calculator to do this automatically, but show the algebra for doing the calculation using your m and b) that should fit the data points well. Plot this function together with the data.
Part 3: The
Half-Life
y = y0
2-t/T.
(Can you explain why?) That is, decay functions can be expressed as exponentials with base 2 by scaling time by the half-life.
Answer each of the following questions in your worksheet.
This lab has been adapted from materials developed by William Barker, Bowdoin College, and David Smith, Duke University for the Connected Curriculum Project with funding from the National Science Foundation. Used by permission.