Water and oxidation state play important roles in terrestrial geochemical cycles. Dehydration of subducting lithosphere induces oxidation and partial melting in the mantle wedge above subduction zones, and storage of water in the form of hydroxyl in high-pressure mineral phases may be an important mechanism for transfer of water to the mantle. Water is important in melt genesis and oxidized metasomatic fluids alter mineral compositions in mantle xenoliths. In crustal settings, metamorphic fluids influence rates of deformation, diffusion and mass transfer of fluid-compatible elements. Identification of the water content of fluids is a first-order problem in understanding the evolution of metamorphic belts because many equilibria that could be used for thermobarometry require knowledge of water activity. Finally, recent interest has focused on the possibility of the existence of a hydrosphere on the very early (>4.3 Ga) Earth, highlighting the need to learn more about the fluid composition and oxygen fugacity of rocks that can provide important information about the early terrestrial oxidation state and water cycle. It is therefore important to quantify the water content of fluids and oxygen fugacities in natural systems, but these variables can be difficult to measure or infer in many rocks. Typical methods of determining oxygen fugacity and water activity involve measuring the composition of coexisting oxygen- or water-buffering assemblages.
We develped a more widely applicable barometer for oxygen fugacity in rocks with known pressure and temperature of formation, based on OH concentration measurements in rutile, a commonly occurring accessory mineral that is nominally anhydrous but can incorporate a significant amount of trace hydroxyl into its structure. This method allows determinations of oxygen fugacity in rocks lacking coexisting oxides.
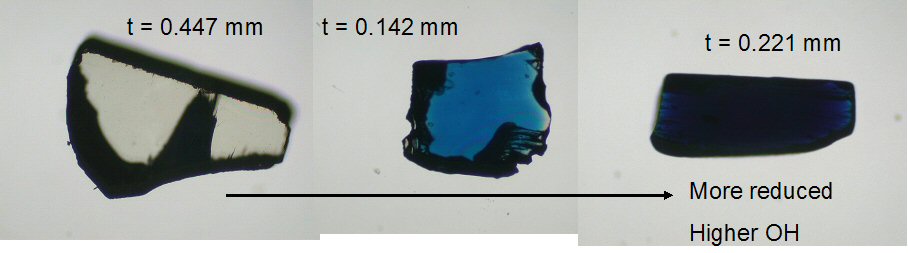
We equilibrate synthetic rutile (see colorless starting material, below left) at different water activities, oxygen fugacities, temperatures, and pressures in a series of piston cylinder experiments. After the experimental runs, the equilibrated rutile changes to a nice blue color! This is due to partial reduction of Ti4+ to Ti3+ in the rutile structure. This redox reaction causes uptake of H+ in the form of hydroxyl into the crystal structure for charge balance. Thus, the deeper the blue, the more "water" the rutile crystal has incorporated!