On October 1, 2003, the Smithsonian's Hope and Blue Heart diamonds were removed from their settings for comparison to the Steinmetz Heart of Eternity, part of the Splendor of Diamonds exhibit at the Smithsonian Institution (King and Shigley, 2003). This event provided a unique opportunity to obtain infrared spectra of these three fancy deep-blue diamonds!
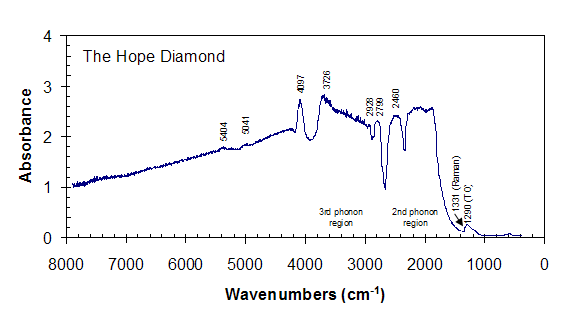
The mid-infrared spectrum of the Hope is typical of deep blue type IIb diamonds (King et al., 1998). There are two principal causes for the absorption features in this energy range: lattice vibrations of the diamond and electronic transitions due to substitution of boron into the diamond structure.
Boron has three valence electrons whereas carbon has four; substitution of a boron atom for a carbon therefore effectively creates a positive “hole” in the structure. As a result, diamonds that are essentially chemically pure except for small amounts (less than 1 ppm) of boron are p-type semiconductors (Smith and Taylor, 1962). The broad absorbance that corresponds to the energy of transition of electrons from the valence band to the conduction band starts roughly at 3000 cm -1 and extends into the near-infrared and red region in the visible spectrum, and is the cause of the blue color of the diamonds. At room temperature blue diamonds have three bands, at 2460 cm-1, 2799 cm-1 , and 2928 cm-1 due to bound hole transitions from the ground state to excited states (The excited states of the holes are lower in energy than the ground state, and sit above the valence band; see Austin and Wolfe, 1956; Smith and Taylor, 1962). Two of these bands (2460 cm-1, 2799 cm-1) are clearly visible in the infrared spectrum of the Hope.